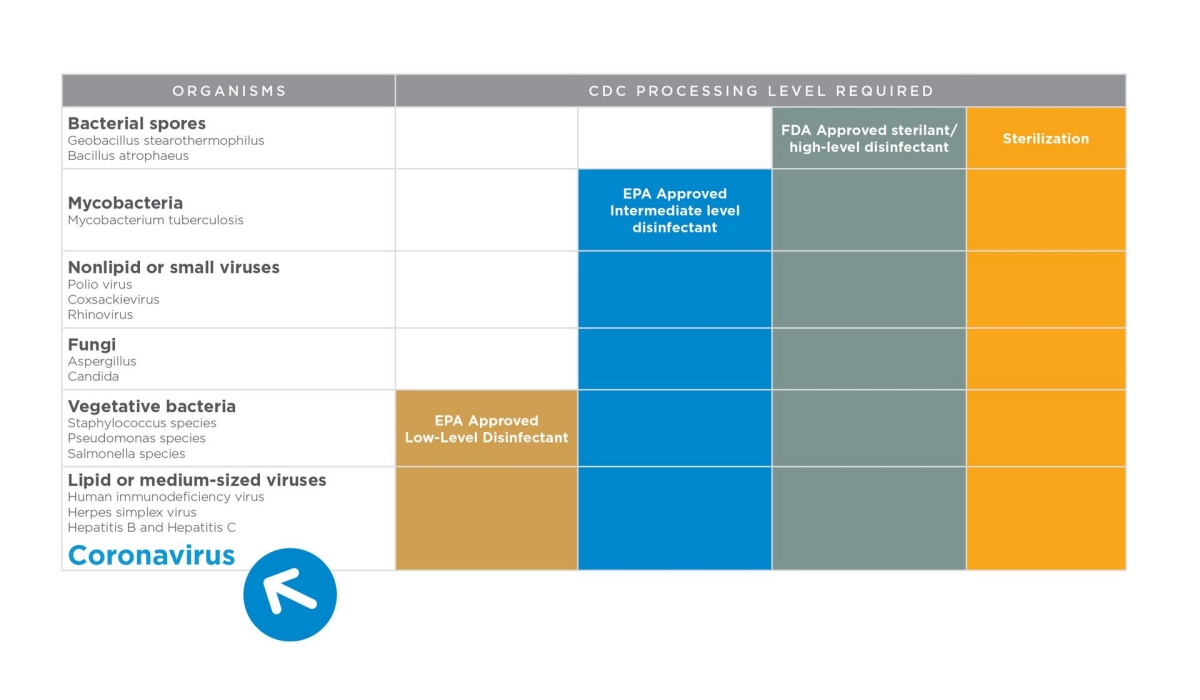
Dental units and equipment are complex systems. Key maintenance may be overlooked when performance is adequate. Maintenance will not only keep equipment at its peak but also protect you and your patients.
After a period of non-use or storage, dental equipment may require maintenance and/or repair. It is important to review the specific manufacturer's instructions for use (IFU) for all equipment and devices. Some considerations for equipment maintenance may include testing, shocking and/or treatment of dental unit waterlines.
Dental Unit Waterlines
"Dental unit waterlines (ie, plastic tubing that carries water to the high-speed handpiece, air/water syringe and ultrasonic scaler) promote bacterial growth and development of biofilm due to the presence of long narrow-bore tubing, inconsistent flow rates and the potential for retraction of oral fluids. Dental healthcare personnel and patients could be placed at risk of adverse health effects if water is not appropriately treated." 2
Dental Unit Waterline Considerations
Follow the facility's dental unit waterline maintenance protocol
Policies and protocols have not been established, create program and train all staff
"All dental units should use systems that treat water to meet drinking water standards (ie, ≤ 500 CFU/mL of heterotrophic water bacteria). Independent reservoirs—or water-bottle systems—alone are not sufficient." 3
Consult with the dental unit and/or waterline treatment product manufacturer for appropriate methods and equipment to monitor and maintain the quality of dental water.
Other Recommendations
Replace/change water filters on devices containing removable filters on devices containing removable filters such as those found on ultrasonic scaling units
"CDC recommends that all dental instruments that use water should be run to discharge water for 20-30 seconds after each patient and for several minutes before the start of each clinic day." 4